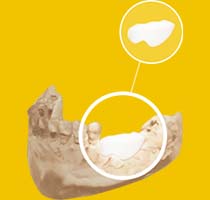
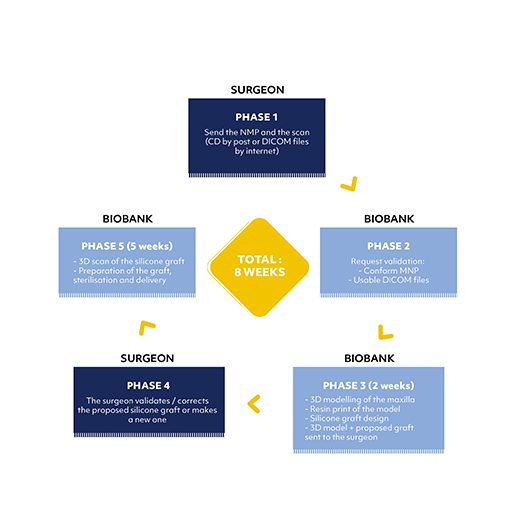
Benefits for the Surgeon :
- Easier planning of the surgery : properties and positioning of the graft can be established in advance on a 3D model
- Surgical time decrease : avoid the cutting/shaping phase necessary for standard bone blocks
- Congruence improvement between the graft and the host site
-
Osteosynthesis facilitated and risks of micro-mobility minimized
- Greater graft / host bone contact surface for better osteoconduction
Benefits for the Patient :
- Reduced opening time of the graft site
- Minimized postoperative complications (inflammation, infection, pain)
- Improved bone volume augmentation in terms of tissue quality and clinical efficiency